Antibody Invented At Charlotte Shows Promise For Pancreatic Cancer Treatment
An antibody that was invented at UNC Charlotte could be used to curb pancreatic cancer relapse and metastasis, a new UNC Charlotte study has found.
Pancreatic cancer is particularly cruel — and deadly. While it is the 10th most common type of cancer, it is the fourth leading cause of cancer death among men and women in the U.S., reflecting its bleak survival rate.
Each November, Pancreatic Cancer Awareness Month calls attention to this silent killer. The cancer has very few symptoms, especially in early stages, meaning it often spreads and forms additional tumors before it is diagnosed. This year’s Annual Report to the Nation on the Status of Cancer, out in October, highlights its grim trends.
Yet, there is hope for improved treatment options. UNC Charlotte researchers are among those leading the search for solutions, including the new research by Mukulika Bose ’22 Ph.D. and collaborators, including Bose’s mentor Pinku Mukherjee, published in the journal Translational Research.
Bose’s groundbreaking research studies TAB004, a patented antibody generated by Mukherjee, who is the Irwin Belk Endowed Professor of Cancer Research and a study co-author.
“We believe,” Bose said, “that this antibody could be used to target cancer cells that remain after surgery on solid tumors, to reduce the cells from colonizing to other organs or tissues. If there is a full-blown tumor, this antibody cannot magically make it vanish. But if there are circulating cancer cells that have detached from the parent tumor and are resistant to chemo and radiotherapy, this antibody can stop these cells from forming secondary tumors.”
Research Targets Secondary Tumors
Patients die due to metastatic secondary tumors more than from primary tumors, making the new study highly impactful. The antibody targets a tumor protein called tMUC1 (t muck one) and blocks its cellular signaling that is critical for tumor progression and metastasis.
“This antibody,” Bose said, “is highly effective at specifically targeting the tumor protein but not the protein that is expressed on normal cells. This is the most novel and significant finding, that it can distinguish between the tumor form of the protein and the protein that is expressed on normal cells.”
Surprisingly, Bose found that the antibody TAB004 was not effective in targeting solid tumors. She instead found that this antibody could precisely target single tumor cells that had just detached from their parent tumor.
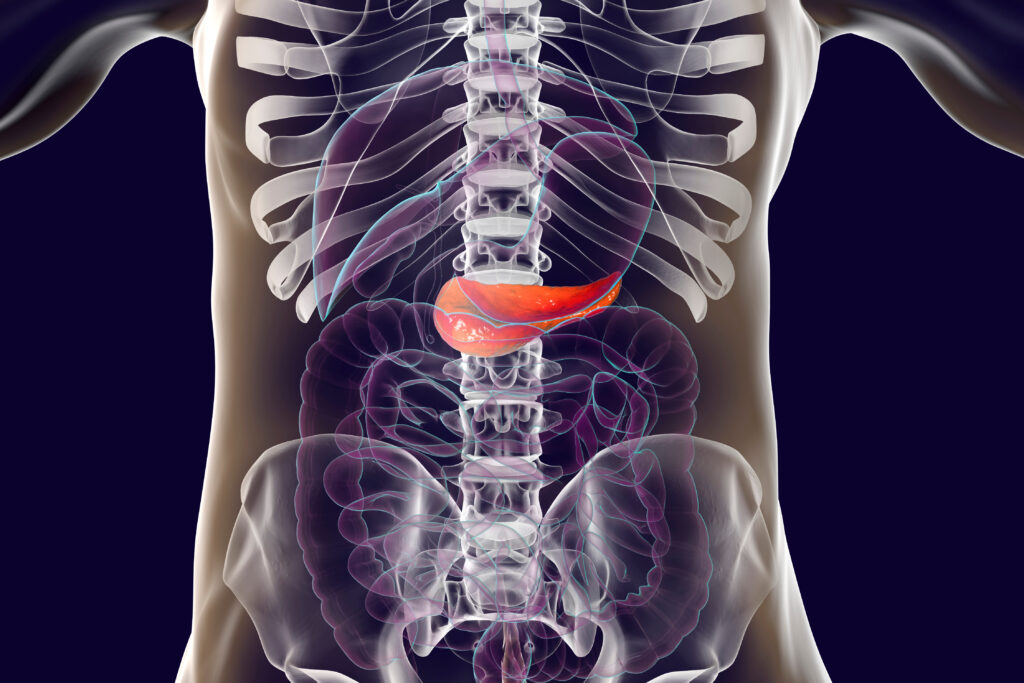
To understand why this was happening, Bose turned to the cell biology of normal healthy cells. Epithelial cells form the tissues that make up our organs, mucus membranes, and skin. To maintain the integrity of these structures, cells form a network of proteins called the extracellular matrix. When cells that interact with this matrix need to undergo programmed cell death, they can induce anoikis, as the cells detach from the extracellular matrix and induces programmed cell death. Anoikis translates in Greek to the word homelessness.
Unlike normal epithelial cells, cancer cells acquire resistance to anoikis, Bose said. This means that when cancer cells detach from the extracellular matrix, they avoid cell death. These cells are then free to travel through the circulatory and lymphatic systems to cause havoc elsewhere in the body.
Cancer Cells Undergo Cell Death
“We used the antibody to destroy anoikis resistance and the ability of the ‘homeless cancer cells’ to remain alive after detaching from the primary tumor,” she said. “They then undergo cell death and cannot form a secondary tumor after treatment with the antibody.”
Cancer cells take two shapes, one that is more rounded, and one that has extensions that are sticky due to the presence of the tMUC1 protein. The second shape, with its stickiness, allows them to grip onto the surface of tissues and organs, somewhat like suction cups. The study found that the antibody TAB004 attaches to the tMUC1 protein and stops the cells from taking the shape with extensions.
“Cells in their rounded shape are not able to grip anywhere,” Bose said. “After a few days of treatment with the antibody, the extended cells round up and finally die.”
Treatment with the antibody showed significant reduction of tumor growth in mouse models. Besides pancreatic cancer, the antibody can target the tMUC1 protein in breast, ovarian and liver cancer cells.
Other possible applications for this antibody as a therapeutic agent include using it in combination with chemotherapy drugs and and as a targeted agent with nanoparticles. More pre-clinical studies, including more mouse models, are needed before the research could move to clinical trials, but this study moves the research closer to the goal of clinical trials.
Researcher Seeks To Outwit Cancer
“My hope,” Bose says, “is that we can outsmart cancer and tame it down to a chronic disease. We should be able to extract the fear from people’s minds, that scientists and physicians together have worked to such a degree that people don’t think it’s the end of the world. That is what motivates me.”
After defending her doctoral thesis in June, Bose joined the Dana-Farber Cancer Institute at Harvard Medical School as a postdoctoral fellow. While at Charlotte, Bose was awarded the 2022 Phi Kappa Phi Dissertation Fellowship, as one of ten students selected nationally for this award and the first student to receive it at Charlotte.
In addition to Bose and Mukherjee, other study authors are Alexa Sanders, Chandrav De, Ru Zhou, Priyanka Lala, Sophia Shwartz, Bhaskar Mitra and Cory Brouwer.
Through innovative research in computational life sciences and health bioinformatics, UNC Charlotte researchers from diverse fields collaborate to prevent and combat threats to human health, reduce health disparities and increase ecosystem vitality.
Words: Lynn Roberson | Images: Courtesy of Bose and Adobe Stock
Antibody Invented At Charlotte Shows Promise For Pancreatic Cancer Treatment
An antibody that was invented at UNC Charlotte could be used to curb pancreatic cancer relapse and metastasis, a new UNC Charlotte study has found.
Pancreatic cancer is particularly cruel — and deadly. While it is the 10th most common type of cancer, it is the fourth leading cause of cancer death among men and women in the U.S., reflecting its bleak survival rate.
Each November, Pancreatic Cancer Awareness Month calls attention to this silent killer. The cancer has very few symptoms, especially in early stages, meaning it often spreads and forms additional tumors before it is diagnosed. This year’s Annual Report to the Nation on the Status of Cancer, out in October, highlights its grim trends.
Yet, there is hope for improved treatment options. UNC Charlotte researchers are among those leading the search for solutions, including the new research by Mukulika Bose ’22 Ph.D. and collaborators, including Bose’s mentor Pinku Mukherjee, published in the journal Translational Research.
Bose’s groundbreaking research studies TAB004, a patented antibody generated by Mukherjee, who is the Irwin Belk Endowed Professor of Cancer Research and a study co-author.
“We believe,” Bose said, “that this antibody could be used to target cancer cells that remain after surgery on solid tumors, to reduce the cells from colonizing to other organs or tissues. If there is a full-blown tumor, this antibody cannot magically make it vanish. But if there are circulating cancer cells that have detached from the parent tumor and are resistant to chemo and radiotherapy, this antibody can stop these cells from forming secondary tumors.”
Research Targets Secondary Tumors
Patients die due to metastatic secondary tumors more than from primary tumors, making the new study highly impactful. The antibody targets a tumor protein called tMUC1 (t muck one) and blocks its cellular signaling that is critical for tumor progression and metastasis.
“This antibody,” Bose said, “is highly effective at specifically targeting the tumor protein but not the protein that is expressed on normal cells. This is the most novel and significant finding, that it can distinguish between the tumor form of the protein and the protein that is expressed on normal cells.”
Surprisingly, Bose found that the antibody TAB004 was not effective in targeting solid tumors. She instead found that this antibody could precisely target single tumor cells that had just detached from their parent tumor.

To understand why this was happening, Bose turned to the cell biology of normal healthy cells. Epithelial cells form the tissues that make up our organs, mucus membranes, and skin. To maintain the integrity of these structures, cells form a network of proteins called the extracellular matrix. When cells that interact with this matrix need to undergo programmed cell death, they can induce anoikis, as the cells detach from the extracellular matrix and induces programmed cell death. Anoikis translates in Greek to the word homelessness.
Unlike normal epithelial cells, cancer cells acquire resistance to anoikis, Bose said. This means that when cancer cells detach from the extracellular matrix, they avoid cell death. These cells are then free to travel through the circulatory and lymphatic systems to cause havoc elsewhere in the body.
Cancer Cells Undergo Cell Death
“We used the antibody to destroy anoikis resistance and the ability of the ‘homeless cancer cells’ to remain alive after detaching from the primary tumor,” she said. “They then undergo cell death and cannot form a secondary tumor after treatment with the antibody.”
Cancer cells take two shapes, one that is more rounded, and one that has extensions that are sticky due to the presence of the tMUC1 protein. The second shape, with its stickiness, allows them to grip onto the surface of tissues and organs, somewhat like suction cups. The study found that the antibody TAB004 attaches to the tMUC1 protein and stops the cells from taking the shape with extensions.
“Cells in their rounded shape are not able to grip anywhere,” Bose said. “After a few days of treatment with the antibody, the extended cells round up and finally die.”
Treatment with the antibody showed significant reduction of tumor growth in mouse models. Besides pancreatic cancer, the antibody can target the tMUC1 protein in breast, ovarian and liver cancer cells.
Other possible applications for this antibody as a therapeutic agent include using it in combination with chemotherapy drugs and and as a targeted agent with nanoparticles. More pre-clinical studies, including more mouse models, are needed before the research could move to clinical trials, but this study moves the research closer to the goal of clinical trials.
Researcher Seeks To Outwit Cancer
“My hope,” Bose says, “is that we can outsmart cancer and tame it down to a chronic disease. We should be able to extract the fear from people’s minds, that scientists and physicians together have worked to such a degree that people don’t think it’s the end of the world. That is what motivates me.”
After defending her doctoral thesis in June, Bose joined the Dana-Farber Cancer Institute at Harvard Medical School as a postdoctoral fellow. While at Charlotte, Bose was awarded the 2022 Phi Kappa Phi Dissertation Fellowship, as one of ten students selected nationally for this award and the first student to receive it at Charlotte.
In addition to Bose and Mukherjee, other study authors are Alexa Sanders, Chandrav De, Ru Zhou, Priyanka Lala, Sophia Shwartz, Bhaskar Mitra and Cory Brouwer.
Through innovative research in computational life sciences and health bioinformatics, UNC Charlotte researchers from diverse fields collaborate to prevent and combat threats to human health, reduce health disparities and increase ecosystem vitality.
Words: Lynn Roberson | Images: Courtesy of Bose and Adobe Stock
RESEARCH OF DR. MENELAOS POUTOUS HIGHLIGHTED BY SPIE
The research of Associate Professor Dr. Menelaos Poutous has recently been highlighted by The International Society for Optics and Photonics due to the valuable insights it offers surrounding the fabrication of diffraction gradings. The paper, published by Dr. Poutous and Dr. Hanshin Lee of the University of Texas at Austin in October of this year, is titled “Reactive ion plasma etched surface relief gratings for low/medium/high resolution spectroscopy in astronomy.” As SPIE explains,
“Today, astronomers seek to observe the faintest and most distant objects possible. Extremely Large Telescopes (ELTs), with apertures in the order of several dozen meters, are the next generation facilities to do so. However, building larger telescopes is only one part of the equation. The other part is the capability of detecting the gathered photons in the most efficient way possible. This is where making all other optical components in astronomical instruments more efficient becomes crucial. One essential component used in modern astronomical science is the diffraction grating. Its role is to spatially spread incoming light into its constituent frequencies, similar to how a glass prism does. Thanks to a precisely engineered structure that leverages the wave-like nature of photons, diffraction gratings can separate light of different wavelengths with very high resolution. When coupled with a telescope and a spectrometer, gratings allow scientists to analyze the spectral properties of celestial bodies.
Motivated by the somewhat stagnant progress made in grating technology over the past decade, researchers Hanshin Lee of the University of Texas at Austin and Menelaos K. Poutous of the University of North Carolina at Charlotte, USA, focused on a completely different way of fabricating diffraction gratings. In their paper, […] they report their success on manufacturing proof-of-concept high-efficiency diffraction gratings using reactive ion-plasma etching (RIPLE), a plasma-based manufacturing technology normally used for semiconductors.”
Dr. Poutous first joined the Department in 2008 as the Senior Scientist of the Microphotonics Laboratory at the Center for Optoelectronics and Optical Communications, and he co-founded the Optical Structured Surfaces Lab in 2013. His research interests include spectroscopy, diffractive micro-optical elements, and artificial optical surfaces and coatings, among other research areas, and he has published more than ninety-five papers in scientific journals and conference proceedings.
Click here to see the original news highlight of the paper on SPIE’s website
2022 Recipient of the Excellence in Leadership Award by the UNC Charlotte Alumni Association and the Black Alumni Chapter
Congratulations to Michelle Pass on being named a 2022 recipient of the Excellence in Leadership award by the UNC Charlotte Alumni Association and the Black Alumni Chapter. In recognition of her accomplishments, she will be honored at an Excellence in Leadership Awards Luncheon on Friday, October 21 at the UNC Charlotte Marriott Hotel and Conference Center. Dr. Pass is the Director of Diversity Equity and Inclusion for the Department of Biological Sciences. She is a long-time senior lecturer, academic advisor, and lab coordinator.
In recognition of her accomplishments, she will be honored at an Excellence in Leadership Awards Luncheon on Friday, October 21 at the UNC Charlotte Marriott Hotel and Conference Center.
2022 EARLY CAREER INVESTIGATOR AWARD OF THE AMERICAN SOCIETY FOR HISTOCOMPATIBILITY & IMMUNOGENICS AND ITS SCIENCE & TECHNOLOGY INITIATIVES COMMITTEE
Dr. Danillo Augusto is the winner of the 2022 Early Career Investigator Award of the American Society for Histocompatibility & Immunogenics and its Science & Technology Initiatives Committee (STIC) The aim of the ASHI Early Career Investigator Award (ECIA) is to identify future research leaders who are committed to conducting impactful basic science research. This program offers ASHI members an award of up to $40,000 for research related to immunogenetics in various clinical disciplines such as transplantation, cancer, autoimmunity, infectious disease, disease association, or pharmacogenomics. Dr. Augusto will receive his award on October 25, 2022 at the ASHI Annual Meeting in Las Vegas, NV.
Dr. Sharon Bullock was selected to participate in the training program of the International Student Exchange Program
Congratulations to Dr. Sharon Bullock who was selected to participate in the training program of the International Student Exchange Program (ISEP). There were only 20 spots for participants in the ISEP-AHEA academy from around the U.S. Dr. Bullock will attend a training institute this Fall 2022 and then work to create a Global Network Learning course for Spring 2023. UNC Charlotte is a member of ISEP — a consortium of universities that allow for exchange of students between institutions. ISEP partnered with an organization called AHEA (American Higher Education Alliance) to create GNL/COIL opportunities between the ISEP member institutions. The ISEP-AHEA academy will first train participants on how to do a GNL/COIL course.
Charlotte Mourns The Loss Of Botanical Gardens Director Emeritus Larry Mellichamp
The Charlotte community mourns the Sept. 12 death of UNC Charlotte Botanical Gardens Director Emeritus Thomas Lawrence (Larry) Mellichamp ‘70, known by many affectionately as Dr. M.
Mellichamp joined the faculty of what was then the Department of Biology (now Biological Sciences) in 1976 after completing a doctoral degree at the University of Michigan. He had earned a bachelor’s degree from UNC Charlotte in 1970, and was active in the development of the Botanical Gardens even as an undergraduate student.
An expert on native wildflowers, trees, shrubs, and carnivorous plants, Mellichamp gave hundreds of talks, taught thousands of students and community members, wrote technical and general audience articles on plants and gardening, and wrote or co-authored six books that serve novice and advanced gardeners, including perhaps the best known, “Native Plants of the Southeast.”
Among his many honors, Mellichamp, who retired in 2014 after almost four decades as director of the Botanical Gardens, was the seventh person ever to receive the prestigious Flora Caroliniana Award from the North Carolina Botanical Garden.


Other honors included the Association of Southeastern Biologists Teaching award, the Tom Dowd Award from the Cullowhee Native Plant Conference, and the International Carnivorous Society Lifetime Achievement award.
Throughout his career, he collaborated with many others to transform the Botanical Gardens into a site for active teaching and learning, and a place of beauty and respite for people on campus and in the broader community. He remained active with the Botanical Gardens following his retirement, providing expertise at the annual plant sales, teaching classes, and offering support in many other ways.

All are invited to leave a tribute on a memorial website. His obituary was lovingly written by daughter Audrey Mellichamp and longtime colleague Paula Gross. A public memorial service will take place in the Botanical Gardens the afternoon of Sunday October 30; details will be posted on the Gardens website as they are available. Memorial donations may be sent to the Foundation of the Carolinas for the Mellichamp Garden Staff Enrichment Fund, 220 North Tryon Street, Charlotte, NC 28202.
Charlotte Scientist Among Select Few To Win Scialog Support For Next-Gen Imaging Technologies
Charlotte researcher Rosario Porras-Aguilar, whose work includes a focus on learning how cancers spread, is one of 21 early career scientists in the United States and Canada to win funding and other support through the Scialog: Advancing BioImaging initiative. Scialog aims to accelerate the development of the next generation of imaging technologies.
Porras-Aguilar, an assistant professor in the Department of Physics and Optical Science, and colleague Arnold Hayer, a biologist at McGill University in Montreal, each will receive $50,000 for their interdisciplinary research project, “High-speed 4D Morphodynamic Analysis of Migrating Cells.”
They form one of the 10 teams chosen for grants through Scialog, with funding provided by Research Corporation for Science Advancement (RCSA), the Chan Zuckerberg Initiative, the Frederick Gardner Cottrell Foundation (FGCF), and Walder Foundation. Their team’s funding specifically comes from RCSA and FGCF.
Their project seeks to answer fundamental questions on cellular migration using the new 4D (four dimensions) quantitative microscopy techniques — which are precise and low-cost — recently invented by Porras-Aguilar’s Charlotte lab. The answers to these questions can help expand understanding of cancer proliferation and cellular evolution.
Looking For Brave Biologists
“One of the most challenging aspects of my work as a microscopist,” Porras-Aguilar said, “is finding collaborators in biology willing to use new imaging techniques. Scialog provides me with the invaluable opportunity to establish collaborations among brave biologists with common research interests.”

Porras-Aguilar’s lab is developing label-free microscopy techniques to obtain quantitative data in three and – with the recent discovery – four dimensions. The innovations harness the optical properties of smart materials to drive applications in microbiology and industry.
Hayer’s research is focused on collective movement of cells, a process with critical importance for development, repair, and disease. The research strives to identify how functional coupling between cells is achieved, through communication across adhesive cell-cell junctions.
Porras-Aguilar also was a 2021 recipient of RCSA’s $100,000 Cottrell Scholar Award, one of 25 teacher-scholars in chemistry, physics, and astronomy recognized for the quality and innovation of their research programs and their potential for academic leadership.
Scialog, which is short for “science + dialog,” offers more to the researchers than funding. Created in 2010 by RCSA, the Scialog format supports research through intensive interdisciplinary conversation and community building around a scientific theme of global importance.
A May 2022 conference in Tucson, Arizona brought together 45 early career chemists, physicists, biologists, bioengineers, and medical imaging specialists. Keynote speakers Brian Pogue, University of Wisconsin-Madison, and Jenn Prescher, University of California, Irvine, set the stage for discussion with talks about their research, current tools and needed breakthroughs in imaging, and what they believe are promising areas for discovery.
Gaining Input On Future Research
“Having an environment where bioimaging is discussed among senior and early career investigators with diverse expertise gave me feedback on my research direction,” Porras-Aguilar said. “It offered the opportunity to establish long-lasting multidisciplinary collaborations, and an open perspective of where the field needs to move forward and what challenges we need to address.”
Teams of two to three fellows who had not previously collaborated competed for the seed funding. They developed new research ideas to bridge their different expertise, methods, and technologies in new ways to enable major advances in bioimaging. They wrote and pitched proposals, competing for the grants.
“Multidisciplinary collaborations create synergies that spark new ideas,” said RCSA President and CEO Daniel Linzer. “In the same way, funding organizations investing in forward-thinking projects like these can work together to expand the horizons of knowledge.”
Research Corporation for Science Advancement is a private foundation that funds basic research in the physical sciences (astronomy, chemistry, physics, and related fields) at colleges and universities in the United States and Canada. The Chan Zuckerberg Initiative was founded in 2015 to help solve some of society’s toughest challenges — from eradicating disease and improving education, to addressing the needs of our communities.
Research Corporation Technologies established the Frederick Gardner Cottrell Foundation in 1998 to provide financial support for scientific research and educational programs at qualified nonprofit organizations. The Walder Foundation was established by Joseph and Elizabeth Walder. Its five areas of focus are science innovation, environmental sustainability, the performing arts, migration and immigrant communities, and Jewish life.
Words: Lynn Roberson
Charlotte Scientist Among Select Few To Win Scialog Support For Next-Gen Imaging Technologies
Charlotte researcher Rosario Porras-Aguilar, whose work includes a focus on learning how cancers spread, is one of 21 early career scientists in the United States and Canada to win funding and other support through the Scialog: Advancing BioImaging initiative. Scialog aims to accelerate the development of the next generation of imaging technologies.
Porras-Aguilar, an assistant professor in the Department of Physics and Optical Science, and colleague Arnold Hayer, a biologist at McGill University in Montreal, each will receive $50,000 for their interdisciplinary research project, “High-speed 4D Morphodynamic Analysis of Migrating Cells.”
They form one of the 10 teams chosen for grants through Scialog, with funding provided by Research Corporation for Science Advancement (RCSA), the Chan Zuckerberg Initiative, the Frederick Gardner Cottrell Foundation (FGCF), and Walder Foundation. Their team’s funding specifically comes from RCSA and FGCF.
Their project seeks to answer fundamental questions on cellular migration using the new 4D (four dimensions) quantitative microscopy techniques — which are precise and low-cost — recently invented by Porras-Aguilar’s Charlotte lab. The answers to these questions can help expand understanding of cancer proliferation and cellular evolution.
Looking For Brave Biologists
“One of the most challenging aspects of my work as a microscopist,” Porras-Aguilar said, “is finding collaborators in biology willing to use new imaging techniques. Scialog provides me with the invaluable opportunity to establish collaborations among brave biologists with common research interests.”

Porras-Aguilar’s lab is developing label-free microscopy techniques to obtain quantitative data in three and – with the recent discovery – four dimensions. The innovations harness the optical properties of smart materials to drive applications in microbiology and industry.
Hayer’s research is focused on collective movement of cells, a process with critical importance for development, repair, and disease. The research strives to identify how functional coupling between cells is achieved, through communication across adhesive cell-cell junctions.
Porras-Aguilar also was a 2021 recipient of RCSA’s $100,000 Cottrell Scholar Award, one of 25 teacher-scholars in chemistry, physics, and astronomy recognized for the quality and innovation of their research programs and their potential for academic leadership.
Scialog, which is short for “science + dialog,” offers more to the researchers than funding. Created in 2010 by RCSA, the Scialog format supports research through intensive interdisciplinary conversation and community building around a scientific theme of global importance.
A May 2022 conference in Tucson, Arizona brought together 45 early career chemists, physicists, biologists, bioengineers, and medical imaging specialists. Keynote speakers Brian Pogue, University of Wisconsin-Madison, and Jenn Prescher, University of California, Irvine, set the stage for discussion with talks about their research, current tools and needed breakthroughs in imaging, and what they believe are promising areas for discovery.
Gaining Input On Future Research
“Having an environment where bioimaging is discussed among senior and early career investigators with diverse expertise gave me feedback on my research direction,” Porras-Aguilar said. “It offered the opportunity to establish long-lasting multidisciplinary collaborations, and an open perspective of where the field needs to move forward and what challenges we need to address.”
Teams of two to three fellows who had not previously collaborated competed for the seed funding. They developed new research ideas to bridge their different expertise, methods, and technologies in new ways to enable major advances in bioimaging. They wrote and pitched proposals, competing for the grants.
“Multidisciplinary collaborations create synergies that spark new ideas,” said RCSA President and CEO Daniel Linzer. “In the same way, funding organizations investing in forward-thinking projects like these can work together to expand the horizons of knowledge.”
Research Corporation for Science Advancement is a private foundation that funds basic research in the physical sciences (astronomy, chemistry, physics, and related fields) at colleges and universities in the United States and Canada. The Chan Zuckerberg Initiative was founded in 2015 to help solve some of society’s toughest challenges — from eradicating disease and improving education, to addressing the needs of our communities.
Research Corporation Technologies established the Frederick Gardner Cottrell Foundation in 1998 to provide financial support for scientific research and educational programs at qualified nonprofit organizations. The Walder Foundation was established by Joseph and Elizabeth Walder. Its five areas of focus are science innovation, environmental sustainability, the performing arts, migration and immigrant communities, and Jewish life.
Words: Lynn Roberson
UNC Charlotte-Led Team Invents New Anticoagulant Platform, Offering Hope For Advances For Heart Surgery, Dialysis, Other Procedures
While blood clotting is important to prevent blood loss and for our immunity, coagulation also can cause health issues and even death. Currently, one in four people worldwide dies from diseases and conditions caused by blood clots. Meanwhile, anticoagulants used to reduce risks can also cause significant issues, such as uncontrolled bleeding.
Now, a new biomolecular anticoagulant platform invented by a team led by UNC Charlotte researcher Kirill Afonin holds promise as a revolutionary advancement over the blood thinners currently used during surgeries and other procedures. The team’s discoveries are reported in the journal Nano Letters, first available online on July 5.
“We envision the uses of our new anticoagulant platform would be during coronary artery bypass surgeries, kidney dialysis, and a variety of vascular, surgical and coronary interventions,” Afonin said. “We are now investigating if there are potential future applications with cancer treatments to prevent metastasis and also in addressing the needs of malaria, which can cause coagulation issues.”
The paper shares the most recent results from three years of collaboration among researchers with the Frederick National Laboratory for Cancer Research (Nanotechnology Characterization Laboratory), University of São Paulo in Brazil, The Pennsylvania State University, and Uniformed Services University of the Health Sciences.
“All this resulted in a massive international and interdisciplinary effort to develop a completely new technology that we think may revolutionize the field and be picked up by other areas of health research,” Afonin said.
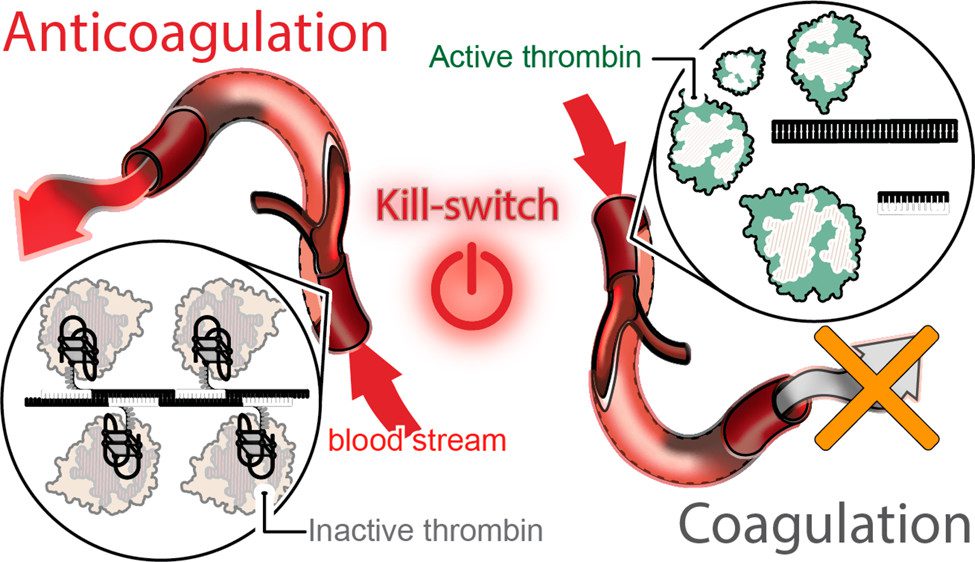
The team’s technology turns to programmable RNA-DNA anticoagulant fibers that, when injected into the bloodstream, form into modular structures that communicate with thrombin, which are the enzymes in blood plasma that cause blood to clot. The technology allows the structures to prevent blood clotting as it is needed, then be swiftly eliminated from the body by the renal system once the work is done.
The fiber structures use aptamers, short sequences of DNA or RNA designed to specifically bind and inactivate thrombin.
“Instead of having a single small molecule that deactivates thrombin,” Afonin said, “we now have a relatively large structure that has hundreds of the aptamers on its surface that can bind to thrombin and deactivate them. And because the structure becomes larger, it will circulate in the bloodstream for a significantly longer time than traditional options.”
The extended circulation in the bloodstream allows for a single injection, instead of multiple doses. The design also decreases the concentration of anticoagulants in the blood, resulting in less stress on the body’s renal and other systems, Afonin said.
This technology also introduces a novel “kill-switch” mechanism. A second injection reverses the fiber structure’s anticoagulant function, allowing the fibers to metabolize into materials that are tiny, harmless, inactive and easily excreted by the renal system.
The entire process takes place outside the cell, through extracellular communication with the thrombin. The researchers note that this is important as immunological reactions do not appear to occur, based on their extensive studies.

The team has tested and validated the platform using computer models, human blood and various animal models. “We conducted proof-of-concept studies using freshly collected human blood from donors in the U.S. and in Brazil to address a potential inter donor variability,” Afonin said.
The technology may provide a foundation for other biomedical applications that require communication via the extracellular environment in patients, he said. “Thrombin is just one potential application,” he said. “Whatever you want to deactivate extracellularly, without entering the cells, we believe you can. That potentially means that any blood protein, any cell surface receptors, maybe antibodies and toxins, are possible.”
The technique permits the design of structures of any shape desired, with the kill switch mechanism intact. “By changing the shape, we can have them go into different parts of the body, so we can change the distribution,” Afonin said. “It gets an extra layer of sophistication of what it can do.”
While the application is sophisticated, production of the structures is relatively easy. “The shelf life is amazingly good for these formulations,” Afonin said. “They’re very stable, so you can dry them, and we anticipate they will stay for years at ambient temperatures, which makes them very accessible to economically challenged areas of the world.”
While the researchers’ work so far has relevance for short-term applications, such as in surgeries, they hope to possibly extend their research into maintenance situations, such as with medications that patients with heart conditions take.
The potential for saving lives and improving health care is a motivator for the team, as is inventing something new, Afonin said.
“We can learn from nature, but we have built something that has never been introduced before. So, we develop and build all these platforms de novo – from scratch. And then we can explain through our platforms what we want nature – or our bodies – to do and our bodies understand us.”
— Kirill Afonin
UNC Charlotte’s Office of Research Commercialization and Development is working closely with Penn State to patent and bring this new technology to market.
Afonin, professor with the Nanoscale Science Doctoral Program in the Department of Chemistry at UNC Charlotte, is the paper’s corresponding author. Other authors are: Weina Ke of UNC Charlotte, Morgan Chandler of UNC Charlotte, Edward Cedrone of the Frederick National Laboratory for Cancer Research, Renata F. Saito of the University of São Paulo, Maria Cristina Rangel of the University of São Paulo, Mara de Souza Junqueira of the University of São Paulo, Jian Wang of Penn State, Da Shi of Frederick National Laboratory for Cancer Research, Nguyen Truong, of UNC Charlotte, Melina Richardson of UNC Charlotte, Lewis A. Rolband of UNC Charlotte, Didier Dréau of UNC Charlotte, Peter Bedocs of Uniformed Services University of the Health Sciences, Roger Chammas of UNC Charlotte, Nikolay V. Dokholyan of Penn State, and Marina A. Dobrovolskaia of Frederick National Laboratory for Cancer Research.
Words: Lynn Roberson, CLAS Communications Director | Images: Lynn Roberson and University Communications | Diagrams: Courtesy of Kirill Afonin